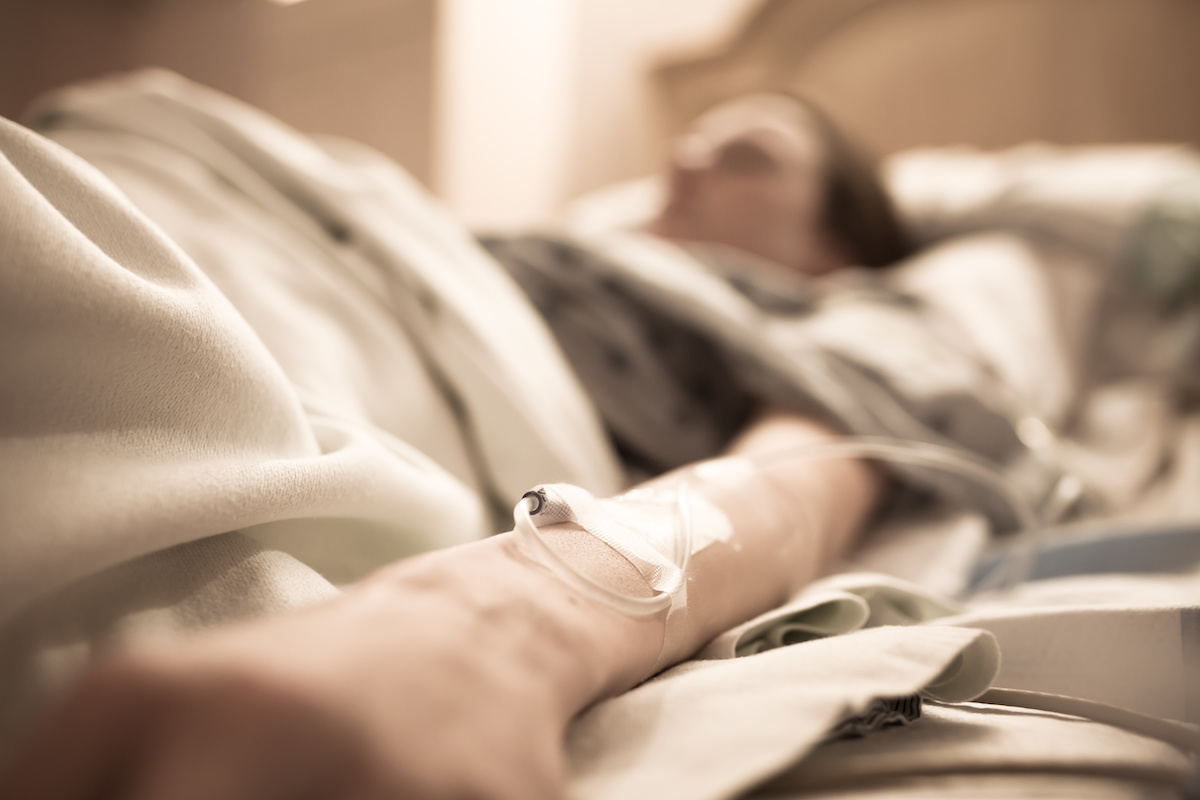
A patient at the Henry Ford Health System in Detroit received a double lung transplant. The damage to the person’s lungs was caused by vaping.
The teen, who wishes to remain anonymous for privacy, was near death when he received a double lung transplant. This procedure was historic as a first for a vaping illness. According to the doctor, it was not like anything he had seen with other patients. His condition was so severe that he went to the top of the donor list, which led to a transplant just a week after making it on the list. As with many other patients, he was first admitted due to symptoms that was first thought to be pneumonia.
More than 2000 people in America have fallen ill due to a mysterious illness caused from e-cigarettes and vaping. At least 40 of them have died from the disease. While the patient wants to protect his privacy and not have his name released, he did want others to know the dangers of vaping.
Risks with a Lung Transplant
A lung transplant is a major operation, and it becomes even more challenging when both lungs are replaced. There is the risk of the body rejecting the lungs because they are seen as a foreign substance. Doctors prescribe anti-rejection drugs, which suppress the immune system to reduce the risk of rejection. They come with risks of their own, including:
- Increase in facial hair
- Stomach issues
- Weight gain
- Diabetes
- Cancer
- High blood pressure
- Damage to kidneys
- Osteoporosis
There is also an increased risk for infection since your immune system has been suppressed.
After the Lung Transplant
The patient will spend several days in the intensive care unit of the hospital. They will use a ventilator to help them with breathing. Tubes are inserted in the chest to keep fluids draining away from the lungs and heart.
Medications will be sent through a tube in the vein for pain control and anti-rejection. After moving out of the ICU, the patient will still stay in the hospital for an extended period. It can be one week to three weeks, depending on how fast recovery is.
Even after the patient leaves the hospital, they must go through frequent monitoring to prevent any complications or to detect and treat them if they occur. The doctor may order lab tests, X-rays, ECG and other testing.
The patient will need to protect themselves from infection, which may mean staying away from others who have colds or not going out in public during cold and flu season. They will need to be alert for any signs of rejection, even though the risk does go down once they are released from the hospital.
Getting a lung transplant means permanent changes and adjustments. The patient will likely need to take suppressant medications for the immune system to keep the body from rejecting the lungs. Doctors will start an exercise routine, which is an important part of rehab. Pulmonary rehab is a combination of exercise and education to improve breathing.
Lung transplants can improve the quality of life for those who have damaged lungs. Some people can live ten years or even longer after a transplant, but about half will only live about five years or less. This vaping illness is serious and is causing significant damage to patients. While a significant number have died, those who survive may have other complications or permanent effects from this illness. Government agencies, as well as patients with the illness and family members, are urging people to stop vaping until a definitive cause is determined.