A factory mix-up of two very different medications has drug manufacturer, AvKare, issuing a recall. The factory packaged two different medications in the wrong bottles. One is an antidepressant, and the other is a medication used to treat erectile dysfunction.
Details of the Recall
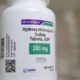
The first medication is trazodone, used as a sedative and to treat depression. The second medication was sildenafil, for erectile dysfunction issues. The medicines were accidentally packaged in the opposite bottles by a third-party manufacturing facility.
The manufacturer is urging anyone with either of these medications to stop taking them. If someone is prescribed the sildenafil for ED, they could notice symptoms, such as blurry vision or dizziness and sedation. For the patient who is taking trazodone, they won’t get the help they need for depression. The medication might interact with other medications they might be on. Sildenafil is not to be taken with nitrates, which are commonly given for heart disease or diabetes.
AvKare has provided notification to the distributors and customers. The company is making arrangements to have all recalled products returned. Both medications are listed at 100mg. Taking sildenafil by accident can cause the blood pressure to drop to a dangerous level. At this point, no reports of adverse reactions have been reported.
To know if your product is part of the recall, you can heck the lot number and expiration date. The sildenafil 100mg tablets are Lot 36884 which expire 03-2022. The trazodone hydrochloride 100mg tablets are lot 36783, and they expire 06/2022. If consumers have questions, they can call 855-361-3993 on Monday through Friday between 8AM and 4PM CST.
The Dangers of the Medications
While AvKARE is based out of Pulaski, Tennessee, the drugs were distributed around the country, according to the US Food and Drug Administration. The risk of adverse side effects is higher for older patients who are already at an increased risk for falls. It can also impair driving.
Patients who have been prescribed the medications that are affected by the recall should contact their medical provider. They will need to get a new prescription to treat their conditions, especially for those who are taking trazodone for depression. They may notice that the medicine they are using isn’t having the same positive impact on their health because they have been given the wrong medication.
Trazodone works to treat insomnia as well as depression. However, it isn’t approved by the FDA for insomnia. It is believed to inhibit the uptake of the hormone serotonin in the brain. Ironically, one of the side effects of this medication is sexual dysfunction, along with headache, constipation or diarrhea. If abruptly stopped, it can lead to anxiety and disturbances with sleep. That’s why it is important to speak with a doctor if you discover you’ve been taking medication which is part of the recall. This medication can also cause drowsiness and the ability of the person to drive a vehicle or operate other types of machinery.
If you have been taking the medication from the recalled lot, you should seek medical attention if you notice any adverse symptoms.