Multiple cases of Fournier’s gangrene have been tied to diabetes medications. This condition is serious and can even be fatal. However, it is a rare condition which usually happens to men past middle age. In fact, lawsuits are being filed for the victims.
What is Fournier’s Gangrene?
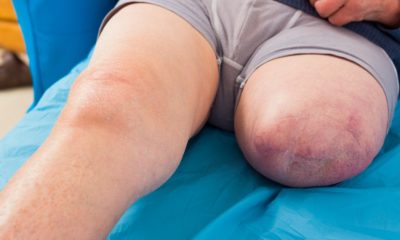
Also known as necrotizing fasciitis, it affects the genital area – mainly of men. The condition is a bacterial infection that is found in the tissues under the part of the skin which is surrounding the blood vessels, fat, muscles and nerves of genitalia.
The most common people to suffer from this condition is males between the ages of 50 and 79. Only about 1.6 of 100,000 are diagnosed each year. It’s known as flesh-eating bacteria because that is what it does.
Fournier’s gangrene or genital gangrene as it’s often referred to develops when bacteria gets into the system from a tear or cut in the skin. It will spread quickly and destroys any tissue it comes into contact with. People who have diabetes are already more susceptible to gangrene because of a lower immunity system and reduced blood flow.
The Link Between Diabetes and Fournier’s Gangrene
In August of 2018, the Food and Drug Administration (FDA) provided a warning that people who were taking type 2 diabetes drugs were at an increased risk for developing this type of gangrene. The type of drugs, which are known as SGLT2 inhibitors will carry new warnings. The drugs included are:
- Canagliflozin
- Dapagliflozin
- Empagliflozin
- Ertugliflozin
And any generic versions.
These drugs work to lower blood sugar levels. They take away any extra glucose in the system by moving it through the kidneys and out of the body in urine. While the medications are designed for people who have been diagnosed with type 2 diabetes, they have shown to be helpful with those who have type 1 diabetes as well.
While the natural incidence rate is low, the number of people diagnosed between March 2013 and May 2018 was significantly higher with 12 cases. All were taking SGLT2 inhibitors. Five of the cases were female and seven were male. The patients were between the ages of 38 and 78 years of age. Fournier’s gangrene is rarely diagnosed in women.
According to reports, the patients developed the condition at an average of about 9 months after they began taking the medication. The actual development was from just seven days all the way to 25 months.
All of the cases required surgery for treatment with one needing skin graft surgery. All needed the infected tissue removed and five had multiple surgeries to take care of the condition. Out of the 12, four of the patients had complications from the condition. These complications included kidney injury, diabetic ketoacidosis and septic shock. One of the patients died. Two others had to go to a rehabilitation hospital.
Data was looked at for other patients who took alternative medications to reduce their blood sugar. During the period between 1984 and 2018, only six cases of the disease were diagnosed. All of the cases were men.
Treatment of the Condition
If a person is taking one of these SGLT2 inhibitors, they should pay attention if they notice swelling, redness or even tenderness of the genital area. If they have a temperature above 100.4 degrees Fahrenheit, they should seek medical attention right away. The condition can worsen quickly.
If the patient is diagnosed with Fournier’s gangrene, they will be treated with antibiotics and may need surgery. They should stop taking the diabetes medications but still monitor blood sugar levels.
The FDA has asked people who experience these or other side effects while taking the inhibitor to contact them and provide information about their case. The agency now requires a warning be included in medicine guides for patients and other information for medical personnel. It was estimated that 1.7 million patients received prescriptions for one of these drugs in 2017.
While genital gangrene has garnered attention with the SGLT2 inhibitors, this is not the only condition to be linked with the medication. Diabetic ketoacidosis is another disease which may be caused by taking the medication. It is another life-threatening condition, which requires treatment right away.
Since diabetics are more susceptible to many health conditions, experts say it is imperative that doctors are aware of these risks and educate patients on them. They need to provide information about recognizing and treating the conditions and advise them of the risk so the patient can make an informed decision about whether to take the medication.