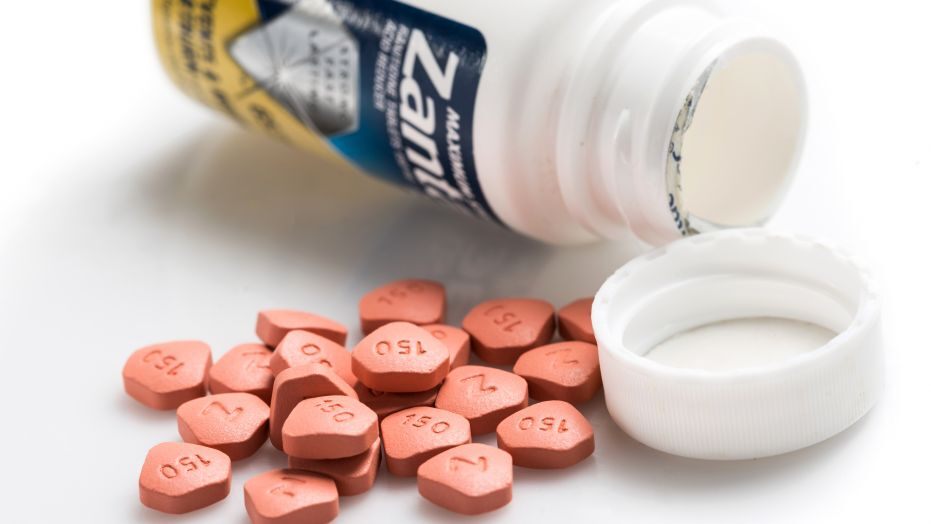
CVS pharmacy is pulling Zantac, an over-the-counter heartburn medication, from the shelf due to evidence of a known carcinogen. The pharmacy is also pulling its own generic version as well.
Traces of N-nitrosodiumethylamine (NDMA) were found in the product. It has also been found in some blood pressure medications. The US Food and Drug Administration (FDA) is reviewing the products to determine if low levels of this chemical pose a risk to people who take the medications.
At this time, the FDA has not recalled the products. There have been no recommendations to stop taking the medications. However, CVS is removing the products as a safety precaution.
Recalls for NDMA
The FDA did recall Valsartan, which is a prescription medication used for high blood pressure as well as heart failure when it was determined to contain traces of NDMA. This chemical is a carcinogen, which was once used to make liquid rocket fuel. Today, it is only used for research.
When people are exposed to high levels of this chemical, they can experience several symptoms, including the following:
- Headaches
- Fever
- Nausea
- Vomiting
- Jaundice
- Loss of function in the kidneys
- Loss of function in the lungs
- Reduced function in the liver
Recalls have been initiated in many countries, including Germany, Sweden, Hungary, Austria, Ireland, Italy, Spain, Croatia and others.
The FDA issued a statement regarding its presence in ranitidine medications, which includes Zantac. It is investigating the occurrence of NDMA in the products to determine the source of the impurity. The agency recommends talking to a doctor before stopping any prescription medications and asking for a substitute. Anyone taking OTC medications with this chemical present should find comparable OTC products for replacement if they don’t feel safe to continue.
Where NDMA is Found
NDMA is also a known contaminant of the environment and can be found in water and some foods, such as vegetables, meat and dairy products.
Ranitidine is a histamine-2 blocker found in both OTC and prescription medications. Its job is to lower the amount of acid that the stomach creates. Products like Zantac are often taken to relieve heartburn, which comes from acid indigestion. Doctors recommend the prescribed versions to treat ulcers as well as gastroesophageal reflux disease.
Anyone who experiences adverse reactions after taking one of these products should report it to FDA. They can complete the report online at the FDA MedWatch website and submit it to the FDA.
There have been no reports of cancer from NDMA in people, but there has been evidence that it caused liver damage which resulted in death from internal bleeding. Animals have suffered liver damage as well from being exposed to the chemical. Mice tested for this chemical had liver and lung cancer along with non-cancerous damage.
While much is unknown about the danger to humans from NDMA, it is safe to assume that all precautions should be taken seriously to prevent possible damage. Anyone taking Zantac and other OTC ranitidine products should be aware of the investigation and consider changing their treatment if a health risk is discovered.