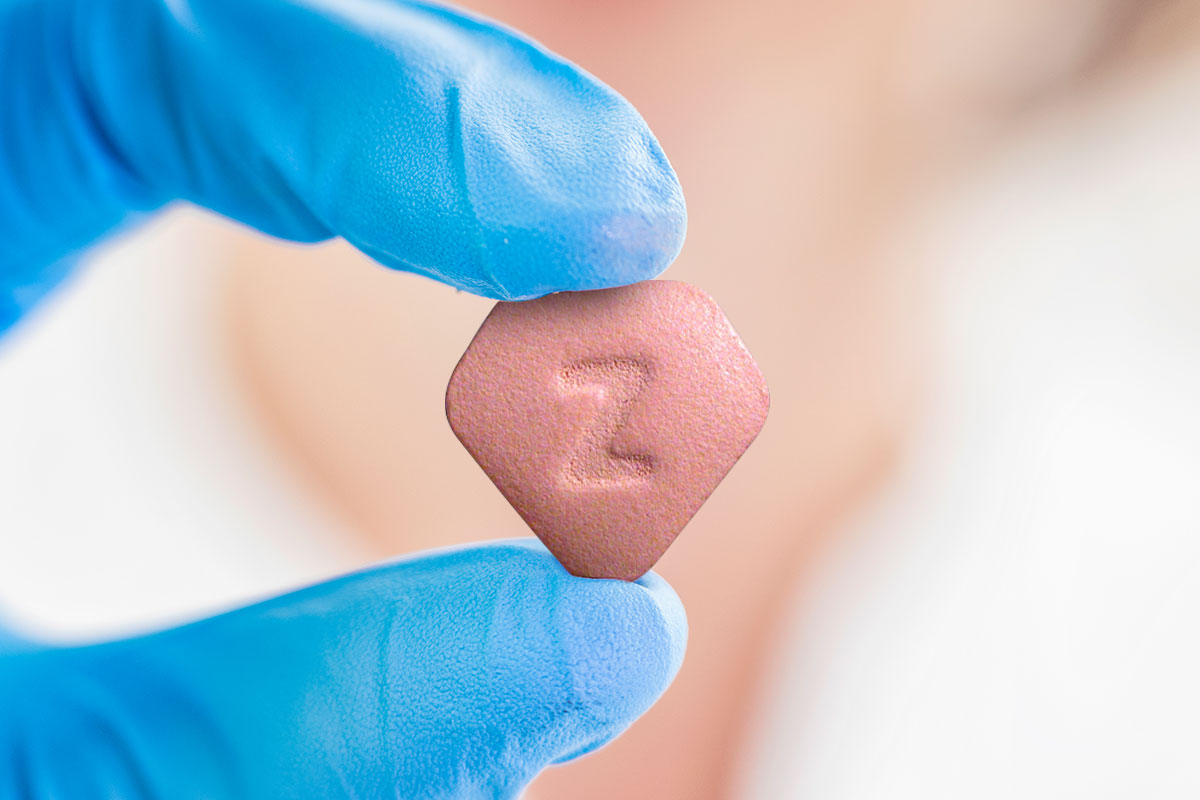
On Wednesday April 1, the US Food and Drug Administration (FDA) ordered that Zantac be pulled from the market. Zantac is a well-known drug used to treat heartburn, but it has been in the middle of controversy for the past six months.
The Risks of Cancer with Zantac
About six months ago, several pharmacies began pulling Zantac and its generic versions from shelves due to a potential carcinogen. Now, the FDA supports this decision and has requested any pharmacies still selling the drug to remove the prescription and OTC versions of the drug, which is basically anything with ranitidine in it. Ranitidine is the active ingredient in Zantac and its generic versions.
Studies show that ranitidine has N-Nitrosodimethylamine (NDMA), which is a contaminant. This substance is also found in some foods, such as cured meats. However, the amount present in ranitidine is above what is allowed by federal regulations.
Back in September 2019, the FDA sent out a warning about the possibility of such an issue. Many large-scale pharmacies, such as Walgreens and CVS, stopped selling Zantac and the equivalents.
According to the FDA, the issue is not with the way it has been manufactured. Instead, it’s the way the ingredient breaks down while it’s being stored. The agency conducted tests of samples, which didn’t have any unacceptable levels of the substance. However, the tests showed that the drug was able to degrade into the substance while sitting in storage. Testing showed it could happen while in tablet form or after it had been consumed by humans.
The problem first came to light in the summer of 2019 when a company known as Valisure did a test on the medication. Valisure is a company that does various safety tests on medications. The results showed the presence of NDMA in alarming levels.
Find New Treatment Options
The FDA is going to be sending out letters to all companies that manufacture ranitidine under any name to have them stop production and pull the product from the market. The manufacturer of Zantac, Sanofi, has already issued a recall in the US and Canada.
The FDA recommends that anyone taking the OTC version of Zantac should switch to another type of heartburn medication immediately. For those who are on a prescription version, they need to talk to their doctors right away about what they can switch to. According to the agency, NDMA has not been found in other medications which act similarly to Zantac, such as Prilosec, Pepcid and Prevacid.
Zantac is most often given for gastroesophageal reflux disease, which is when the acid from the stomach backs up into the esophagus. The medication may also be given for peptic ulcers and Zollinger-Ellison syndrome, which is when the stomach produces too much acid. It mainly comes in two forms, Zantac 75 and Zantac 150. The medication reduces the amount of acid the stomach produces.
NDMA doesn’t cause cancer immediately, but it can build up in the system over time. The risk is long-term if a person continues to take this medication while also being exposed from other ways.